VGXI’s Expanded Manufacturing Capacity Addresses Plasmid DNA Supply Chain Challenges
Plasmid DNA (pDNA) is an essential part of the supply chain for advanced therapies and personalized medicine, including gene therapies, mRNA therapeutics, DNA vaccines, and cell therapies. Yet, the surging demand in the industry far surpasses the current domestic supply capacity. Prolonged wait times and hindered progress are the result. Since its establishment in 1997, VGXI has become a leader in plasmid DNA manufacturing and production, delivering high-purity pDNA products from pre-clinical research through cGMP grade for clinical trials and commercialization. With over 300 batches of clinical-grade cGMP plasmid DNA manufactured totaling 3,250+ grams for clinical trials across the United States, Europe, Asia, Canada, and Australia, VGXI continues to be a trusted partner in advancing life-changing medical research and innovation worldwide.
To meet the demands of tomorrow’s mRNA therapeutics and other advanced therapies, VGXI made strategic investments to build a new state-of-the-art 120,000ft² headquarters and manufacturing facility. The new facility greatly expands plasmid production capacity and process development capabilities. Situated on a 21.5-acre parcel located within Deison Technology Park in Conroe, Texas, VGXI officially opened the new facility in October of 2022. When combined with The Woodlands facility at Research Forest Drive, VGXI’s current state of operational manufacturing space is 160,000ft².
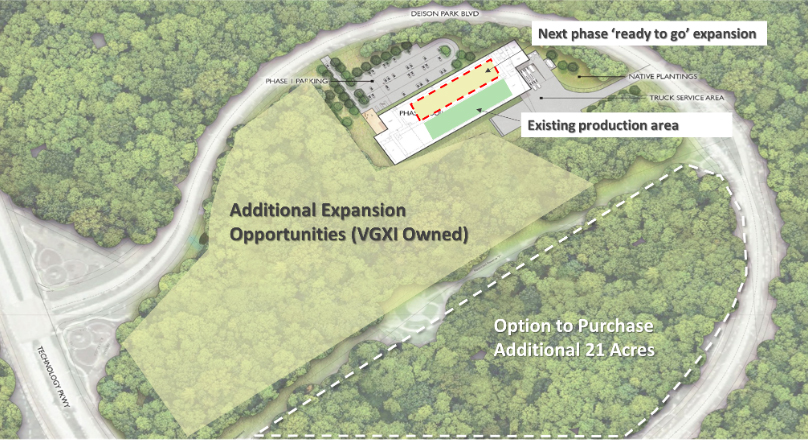
Our 5X expansion since 2022 mirrors the growth trajectory of personalized medicines. Coupled with our roadmap to develop the additional 21 acres, which will add an additional 500, 000+ft² at Deison Technology Park, we solidify our position as the provider of the world’s largest plasmid DNA manufacturing capacity.
Patent Plasmid Manufacturing Processes Boost Efficiency and Reduce Costs
VGXI’s extensive expertise spanning over two decades in pDNA manufacturing has led to the development of advanced technologies and highly efficient and optimized platform processes that enable us to produce high-purity plasmids at scale, while simultaneously reducing costs and accelerating timelines for our clients.
At the core of our platform is our patented AIRMIX® Technology, which gently separates plasmids from cellular debris with minimal shear, preserving plasmid supercoil integrity.
At the core of our platform is our patented AIRMIX® Technology, which gently separates plasmids from cellular debris with minimal shear, preserving plasmid supercoil integrity. This method is significantly more efficient than conventional lysis methods, reducing unit operation time to just a few hours. Moreover, this gentler approach results in pDNA with 10X less genomic DNA contamination, significantly streamlining downstream plasmid purification. Additional advancements made in chromatography steps further reduce set-up and production time leading to substantial cost and time savings overall without sacrificing pDNA quality.
We strive to set new industry standards by pushing the boundaries of what is possible. By continuously improving our technologies and processes, we have gained industry-leading efficiencies in manufacturing and purification so we can deliver cGMP plasmid DNA much faster than current standards. Strategically focused on pure-play pDNA portfolio, research and development, and scientific innovations to drive safety, yield, and cost efficiencies, VGXI’s current capabilities and roadmap for future buildouts both domestically and abroad are unparalleled. We aim to continue leading the way to seamlessly support the rapid growth of next-generation therapeutics and advance breakthroughs that will improve the quality of life for individuals worldwide.


