Knowledge Center
VGXI’s Plasmid DNA Knowledge Center is the resource hub where you will find catalogs, brochures, white papers, and other materials that describe our pDNA products, services, and provide insight into advanced therapies development and commercialization. Discover what makes VGXI a leading-edge Plasmid DNA CDMO in the industry and learn how we can help you advance your therapeutic programs with our products and services.

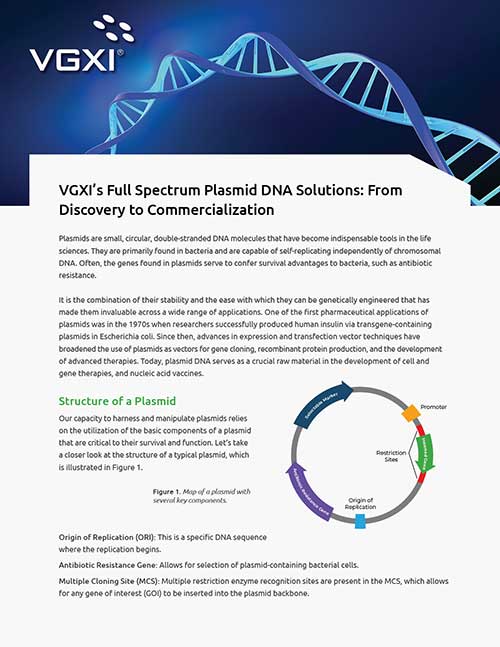
VGXI Primary Catalog 2025
VGXI is a leading-edge global CDMO dedicated to revolutionizing the biopharmaceutical industry with top-tier nucleic acid-based products and services. Download the catalog to learn more.

Pre-Clinical Plasmid Production
VGXI’s Pre-Clinical Plasmids are high purity and low endotoxin specifically designed for pre-clinical development of advanced therapeutic modalities. Download the flyer to learn more.
-Plasmid-Production.jpg)
Highly Documented (HD) Plasmid Production
As your project transitions from pre-clinical phases to early-stage clinical evaluation, plasmid DNA with higher compliance levels is required by regulatory authorities. Download the flyer to learn more.

GMP Plasmid Production
Decades of plasmid DNA production expertise and patented manufacturing technology converge to deliver plasmid production services designed to surpass industry standards. Download the flyer to learn more.

VGXI’s Analytics Program
For the first time ever, our comprehensive analytics package is available as a standalone service to fast-track your development. Download the flyer to learn more.

VGXI Capabilities
VGXI is rooted in over two decades of manufacturing expertise, leading the way in revolutionizing the biopharmaceutical industry. Download the flyer to learn more.

CDMO Expertise Production
VGXI is a leading-edge global CDMO dedicated to revolutionizing the biopharmaceutical industry with top-tier nucleic acid-based products and services. Download the flyer to learn more.

GMP Fill/Finish Service
VGXI addresses this challenge by offering in-house GMP aseptic fill/finish services specifically designed for parenteral products. Download the flyer to learn more.
Rapid TAT Production
In the fast-paced and dynamic landscape of advanced therapies and DNA-based vaccines, getting a program from pre-clinical to first-in-human studies quickly is a strategic advantage. Download the flyer to learn more.