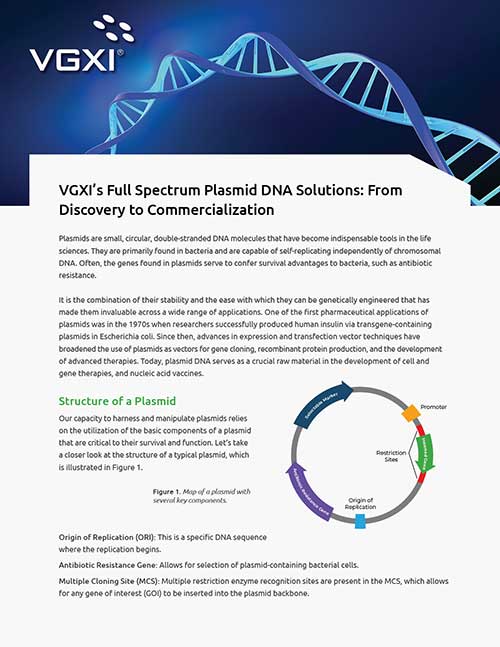
About VGXI, A Plasmid DNA CDMO
VGXI is a leading-edge Contract Development and Manufacturing Organization (CDMO) specializing in the production of plasmid DNA products with a proven track record of success in manufacturing challenging plasmid DNA products under cGMP conditions with exceptional quality, yield, and deliverability. Our industry-leading pDNA manufacturing expertise and ingenuity, coupled with our patented manufacturing process and state-of-the-art facilities, enables VGXI to deliver tailor-made manufacturing solutions to meet our clients’ specific requirements.
From small-scale to commercial production, our consistent Plasmid DNA manufacturing process ensures unparalleled quality at all stages of development, providing end-to-end solutions for every program. Our client’s success is our priority, and we are dedicated to delivering top-notch CDMO Plasmid DNA service and products to meet the highest quality and regulatory standards in the industry.
Our Mission
Our Values
Our Culture
Our Mission
The name VGXI stands for: Vaccine and Gene therapy manufacturing eXcellence in Industry. This embodies our commitment to manufacturing excellence. Our mission is to revolutionize the global pharmaceutical industry by providing best-in-class DNA products and services to support biopharma clients worldwide.
Our Values
Quality is the driving force behind all that we do. Unparalleled commitment to excellence. Accountability for our decisions, actions and results. Leadership to provide purpose, direction, and motivation. Innovation to advance processes and products. Team-oriented to work as a unified team towards a shared objective. You, our clients, are the foundation of our success, and we strive to reflect these values in all that we do.
Our Culture
Our distinct backgrounds and perspectives fuel innovation and drive success, both individually and as a team. We foster a culture of acceptance and inclusion to cultivate a diverse workforce. Our commitment to diversity empowers us to achieve our mission to produce life-saving therapies for patients.